What is Ammonium Bicarbonate?
Know what is ammonium bicarbonate from physical properties and chemical properties
Ammonium bicarbonate is a white compound that appears white at room temperature, granular crystals, and has a trace amount of ammonia odor. It should be noted in preservation because ammonium bicarbonate is a carbonate that reacts with the acid to form carbon dioxide. Thereby, ammonium hydrogen carbonate is deteriorated, so it cannot be placed together with the acid.
Under normal circumstances, the storage of ammonium bicarbonate should be carried out in a cool and dry condition. During storage and transportation, attention should be paid to moisture, rain and sun exposure. It should not be mixed with oxidants, acids or chemicals.
Ammonium bicarbonate can be used as fertilizer for crops
In the countryside, the characteristics of ammonium bicarbonate and acid are often used to react. In the sealed space, ammonium bicarbonate is placed at a high place, and dilute hydrochloric acid is added. After the reaction, sufficient carbon dioxide can be generated for plant photosynthesis, thereby increasing vegetable yield. The ammonium chloride formed by the reaction can also be reused as a fertilizer.
Of course, ammonium bicarbonate can also be used as a nitrogen fertilizer. It can provide ammonium nitrogen and carbon dioxide required for plant growth. After being applied to the soil, ammonium ions and carbon dioxide contained in ammonium bicarbonate can be directly absorbed by plants. It will cause some residual soil and has little effect on the quality of the soil.
Ammonium bicarbonate as a fire extinguisher
Ammonium bicarbonate is also often considered to be an excellent material for making fire extinguishers. Ammonium bicarbonate and aluminum sulfate can be used as a fire extinguishing material for carbon dioxide, alumina, and sodium sulfate. because the carbonate in ammonium bicarbonate is a weak acid anion, aluminum. It is a weak base cation, and the two ions mutually promote the double hydrolysis to form a foam which can effectively extinguish the fire.
Ammonium bicarbonate as a leavening agent in food
Ammonium bicarbonate was first used as a leavening agent. It was made from the reindeer’s horns on the ground. It was also one of the earliest baking powders. Before the emergence of baking soda and other baking powders, Europeans often made ammonium bicarbonate. Bread and biscuits, in which ammonium bicarbonate is used as a leavening agent in foods, during which the ammonium bicarbonate decomposes and releases ammonia and carbon dioxide. The fluffy gas reacts with the fluffy acid to make the food more fluffy and Elasticity, because the biscuits made of ammonium bicarbonate do not have any ammonium residue, and can also ensure the crispness of biscuits to the greatest extent, so some Europeans have retained such a habit.
Before the advent of modern Chinese baking powder, it was often used to make almond biscuits and steamed buns. Although there were astringency and ammonia smell during the baking process, it quickly disappeared without leaving any flavor.
Another name for ammonium bicarbonate: starter
In addition to the leavening agent, ammonium bicarbonate has another name, which is a starter. According to the EU’s specialty food ingredients, there are 10 kinds of starter, including E341 calcium phosphate, E343 magnesium phosphate, E450 DIPHOSPHATES, E500 sodium carbonate, E. 500 I sodium carbonate, E 500 II – sodium bicarbonate, E 500 III – sodium-sesquicarbonate, E 541 – sodium aluminum phosphate, acidic, E 574 – gluconic acid, E 575 – Glucono-delta-lactone.
They affect the food through its ability to form carbon dioxide during the baking process, most of which act to ventilate the dough during the baking process, increasing the elasticity and viscosity of the dough to achieve the optimum volume of the product.
Method for synthesizing ammonium hydrogen carbonate
As shown in the figure below, the compressed carbon dioxide is passed into concentrated ammonia water to maintain the pressurized state of carbon dioxide, and the solution is cooled, crystals are precipitated, and separated by centrifugation, and dehydrated to form ammonium hydrogen carbonate crystals.
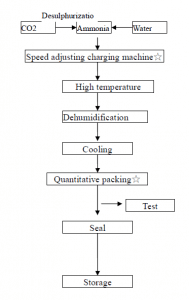
Some typical small nitrogen fertilizer plants in China use anthracite as raw material to prepare semi-water gas. The latter enters the carbonization tower after the hydrogen sulfide is removed, and the mixture of nitrogen, hydrogen and carbon dioxide obtained in the pressure conversion reaction system enters the carbonization tower. About 17% of ammonia water reacts to form ammonium bicarbonate crystals, which are separated by a centrifuge to obtain ammonium bicarbonate product.
Precautions when using ammonium bicarbonate
If you accidentally touch the skin with a certain concentration of ammonium bicarbonate, first remove the contaminated clothing, rinse with running water, accidentally contact with the eyes to wash with a certain concentration of saline, if necessary, you must seek medical attention immediately, if If you accidentally inhale the mouth, you should leave the site to fresh air. If you have difficulty breathing, you should seek medical treatment immediately after oxygen. If you accidentally eat, you must drink enough water, and then seek medical attention immediately after vomiting.
Synonyms: ammonium bicarbonate; carbonic acid, monoammonium salt; monoammonium carbonate; acid ammonium carbonate; ammonia hydrogencarbonate; antler; AmBic;













